Super Hi-Sol
In order to improve the ambiguous effect of hydroxycitric acid (HCA) in slimming body figure, HCA from the fruit of Garcinia atroviridis was extracted with a proprietary technique and incorporated into a formulation containing 70% HCA in the form of water-soluble calcium salt. This formulation (patented in Thailand as Super-Hi-Sol) was packed in sachets, each containing 1.65 g of the formulation (equivalent to 1.15 g of HCA ). For the clinical trial treatment of overweighed females, one sachet was dissolved in water (200 ml.) and taken before meal.
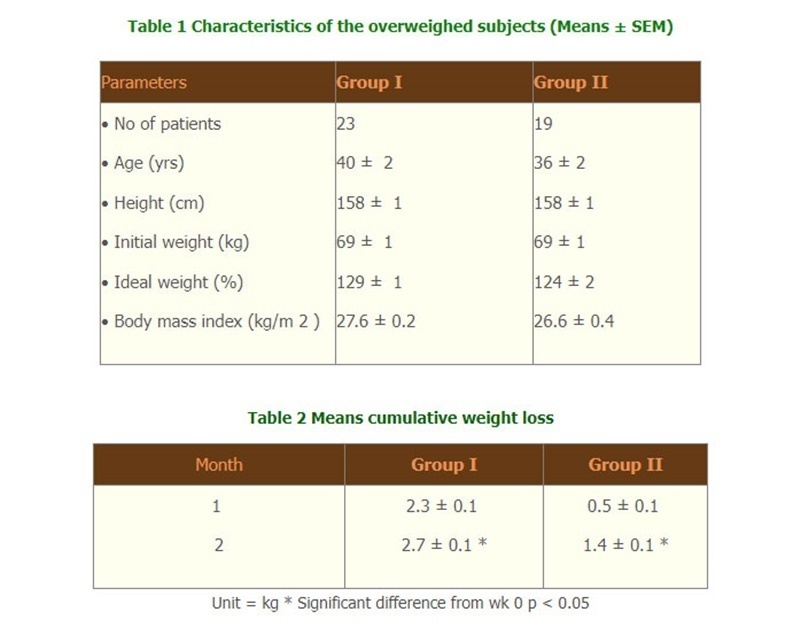
The trial was performed on overweighted Thai patients. The inclusion criteria were: female patients of 18-75 years old, overweighted with body mass index (BMI) over 25 kg/m 2 . Exclusion criteria included diseases of endocrine origin e.g., hypothyroidism, Cushing's syndrome, type I or type II diabetes, serious systemic or psychiatric illnesses, pregnancy, lactating women or desire to become pregnant, and having taken drug for treatment of obesity during the last 3 months. The subjects were divided into 2 groups (Group I, n=23, Group II, n=19). Subjects in group I received one Super Hi-Sol sachet while group II received one placebo sachet before meal, three times daily for 2 months.
The dietary program was recommended to all patients. Throughout the study the energy prescripition was approximately 1,000 kcal/day which provided 53 g. protein, 33 g.fat, and 125 g. carbohydrate.
To assess the efficacy and safety of Super Hi-Sol, we measured body weight which was evaluated weekly in the first month, and every other week in the second month. Anthropometric parameters including triceps, biceps, subscapular and supra-iliac skinfold thickness were measured by Harpenden caliper every month and calculated for percent of body fat from Durnin. We also measured body fat by TBF-511 Body fat- Monitor/Scull (TANITA®) and by BIA (Body stat 1500®).
To assess the safety profiles, we measured blood pressure, hematological and biochemical parameters and serum lipid profiles at the beginning and the end of the program. Each subject answered a questionnaire about compliance and adverse event at each visit.
* The principal investigator, C. Roongpisuthipong, MD, FRCPT, ABN, MCN, is a Professor of Medicine in Ramathibodi Hospital
+ Super Hi-Sol samples were provided by Professor Dr. Pichaet Wiriyachitra, Asian Nutraceutical Centre, 1 st Fl. Loxley Building , 102 Na Ranong Rd. , Klongtoey, Bangkok 10110
All subjects signed the inform consents before starting the project.
- Statistical analysis All the evaluations conducted in the study were recorded in an appropriate case-record form for each subject. The statistical analyses were performed concerning efficacy and safety of Super Hi-Sol parameters. The results were expressed either in absolute values or percentage (%) with the mean and standard error of the mean (SEM). The comparisons were made by using Student t-test.
- Results We excluded 2 subjects in group I and 6 in group II because they did not return for the second week follow up and refused regular contact. The data which were calculated included 23 cases in group I, and 19 cases group II. Characteristics the two groups are shown in table 1. No significant difference was seen between the two groups. Mean?SEM cumulative weight loss after 1 m was 2.3?0.1 kg. in group I and 0.5?0.1 kg. in group II. The mean additional weight loss of 1.8 kg. seen with group I was highly significant (P < 0.05) from group II. (Table2)
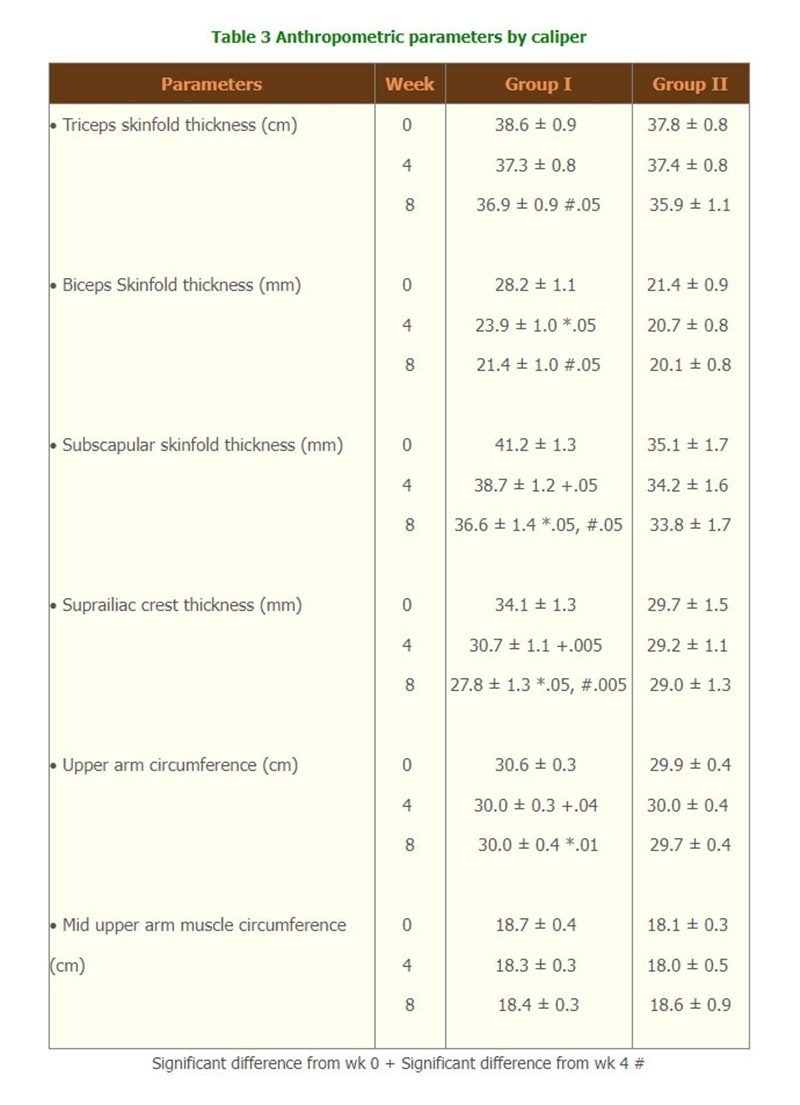
- According to the measurement by Harpenden caliper. Group I showed a gradual decrease in triceps, biceps, subscapular and suprailiac crest skinfold thickness and upper arm circumference with significant difference from those of week 0, except for mid upper arm circumference.(Table 3)
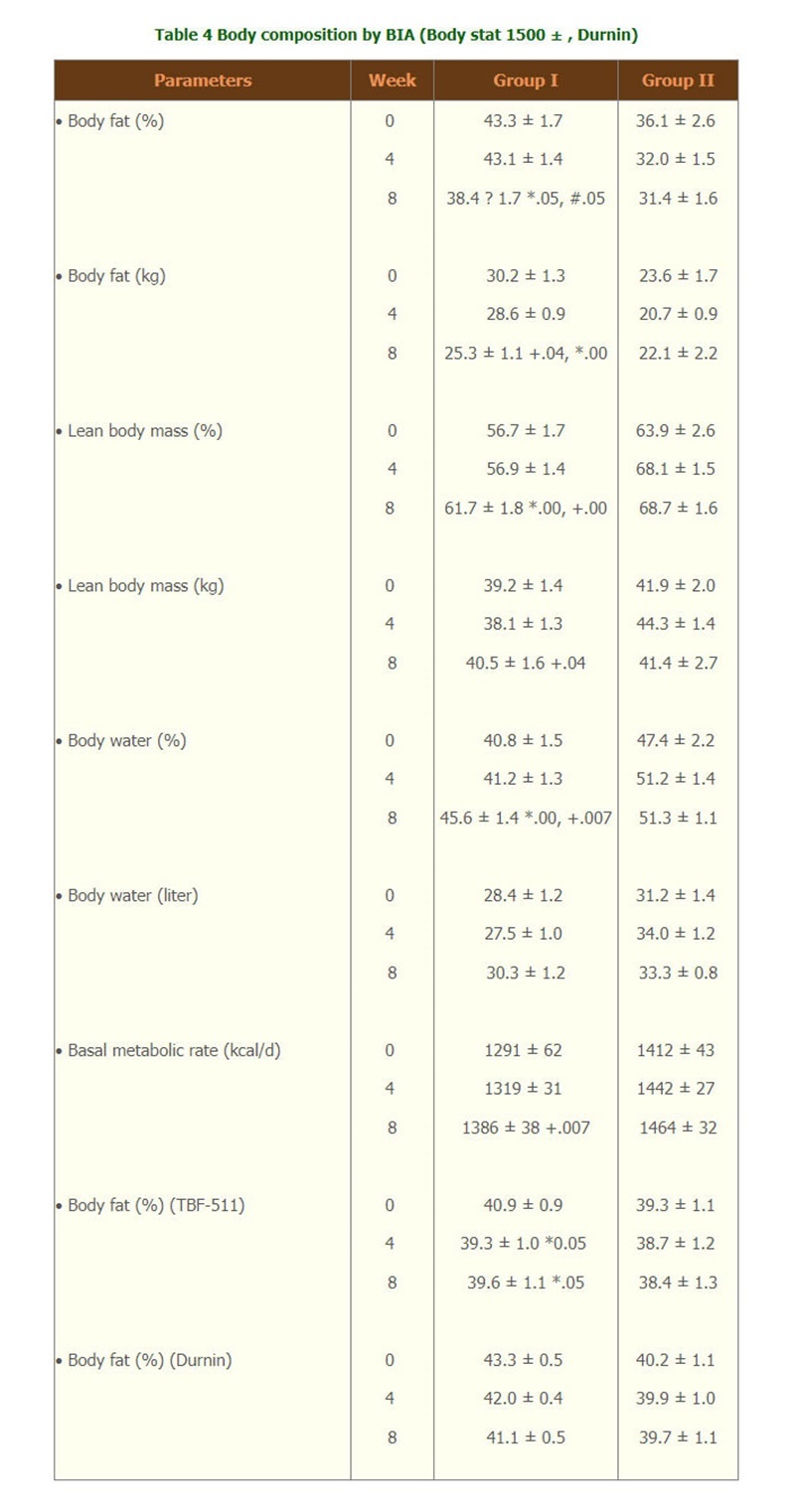
- With the measurement of BIA by 1500 Body stat ? , or TBF-511 , or calculated body fat from Durnin, only group I showed that the weight loss was from body fat which was significantly decreased at month 2 by BIA and Durnin and percent body fat decreased at wk4, wk8 were seen by TBF-511. (Table 4). At week 8 lean body mass and body water were significantly increased from week 0 by BIA. Body cell mass was maintained throughout the study period. The metabolic rate was significantly increased at week 8 form week 0 only in group I. There were no significant difference of all body composition parameters in group II.
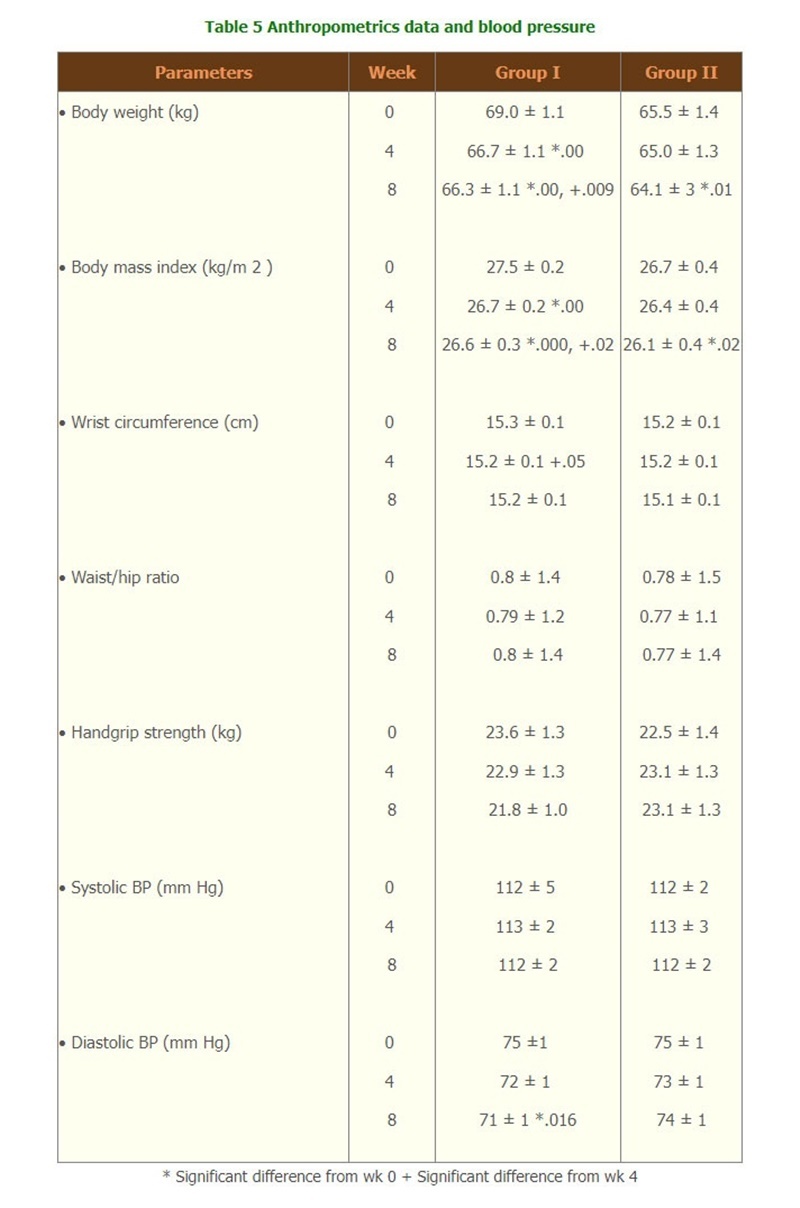
- Body weight of group I was significantly decreased, starting form week 2, throughout the treatment period whereas in group II body weight was significantly decreased at week 8. Waist and hip circumferences were significantly decreased only in group I at week 4. The waist/hip ratio and hand grip strength were not changed in both groups. (Table 5)
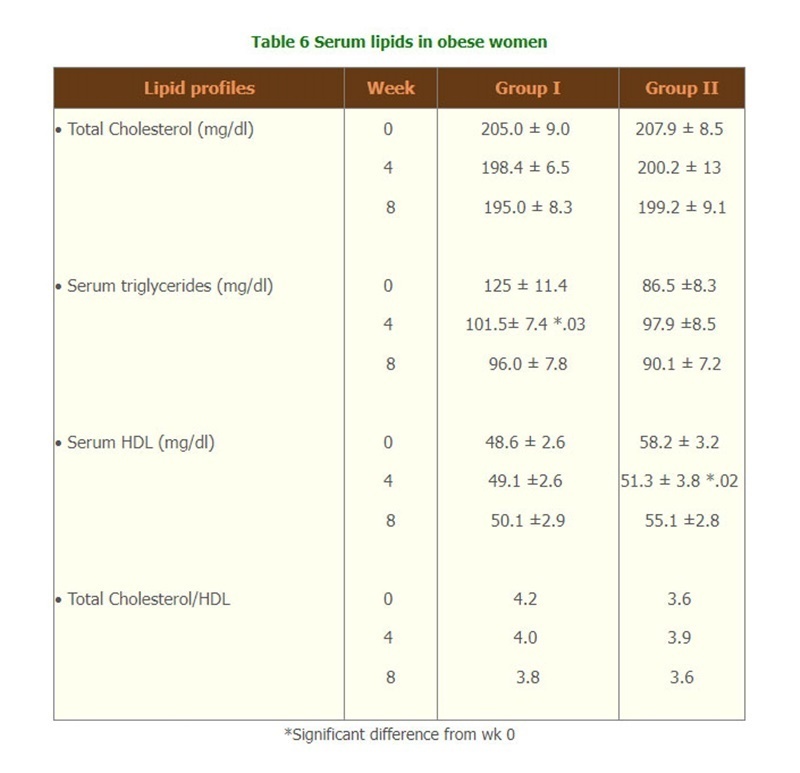
- There were significantly changes of the lipid profiles of Triglyceride level and Total cholesterol/HDL (Table 6) but there were no change of biochemical tests, and hematological parameters, compliances and side effects of both groups throughout the study. (Table 7,8 and 9)
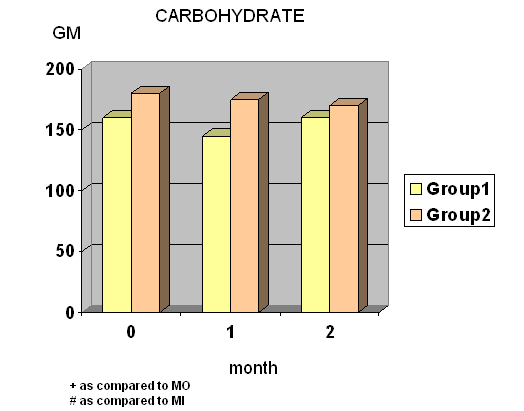
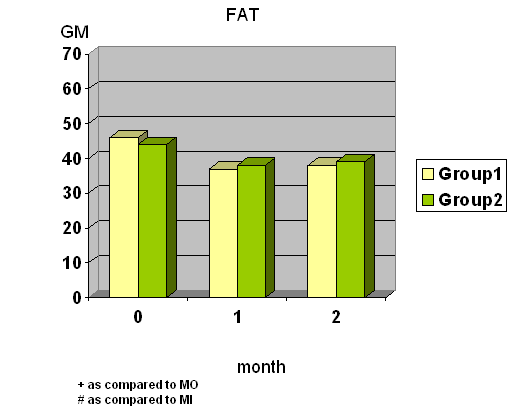
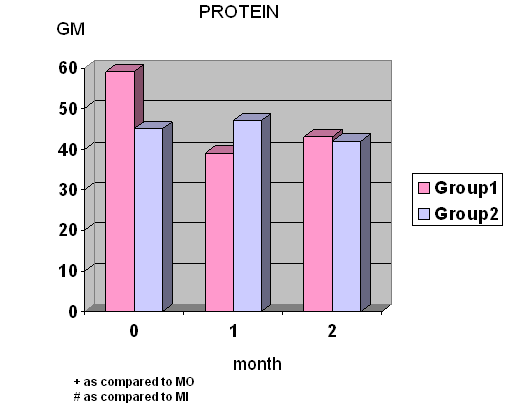
- Total calories, fat and protein intake were significantly decreased at wk 4 and wk 8 in group I whereas total calories, carbohydrate and protein intake were significantly decreased at wk 8 in group II. (Fig.1) Diastolic blood pressure was significantly decreased at wk 8 only in group I.
- Conclusion In our study, we found that Super Hi-Sol has the effect on lowering BW by decreased calories intake, especially of protein and fat intake.
- Body fat was decreased whereas the lean mass maintained. The serum level of Tg and diastolic BP were significantly decreased.
- There was no side effect and no change of hematological parameters and biochemical values.



Ref: Asia Pac J Clin Nutr 2007;16(1) :25-29
Original Article Reduction of adipose tissue and body weight: effect of water soluble calcium hydroxycitrate in Garcinia atroviridis on the short term treatment of obese women in Thailand